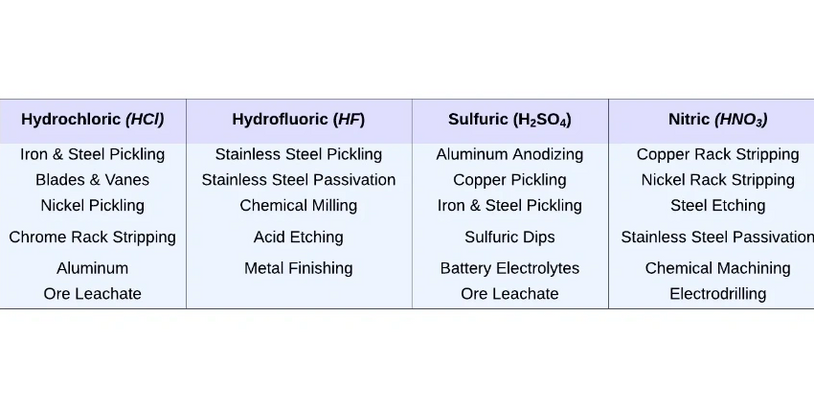
What Is Diffusion Dialysis (DD)?
Diffusion dialysis, also known as concentration or natural dialysis, is a process that uses ion-exchange membranes to separate substances based on concentration gradient. This method has been utilized for separating and recovering acid and alkali waste solutions in an economical and eco-friendly way.
To date, diffusion dialysis has been effectively used to extract and reuse acids and alkalis from the waste streams generated by various industries such as steel production, metal refining, electroplating, regeneration of cation exchange resins, non-ferrous metal smelting, aluminum etching, and tungsten ore smelting.
How Does Diffusion Dialysis Work?
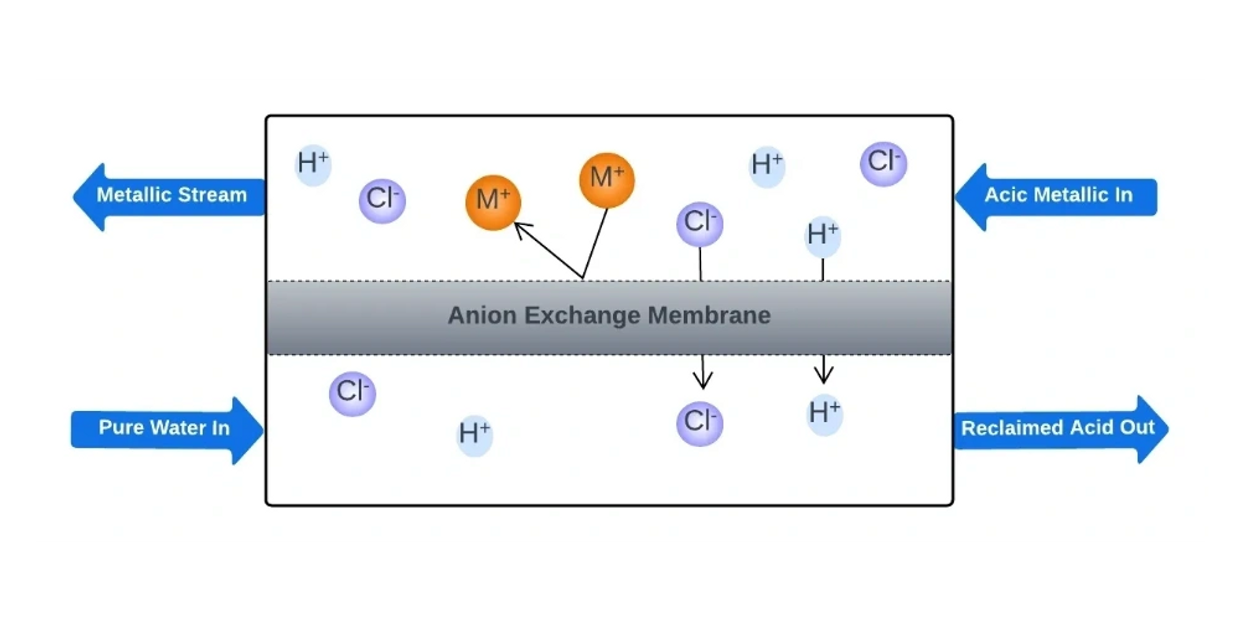
The process of diffusion dialysis (DD) relies mainly on the concentration gradient to drive the transport of ions, while adhering to the Donnan principles of co-ion rejection and preservation of electrical neutrality. For example, in the separation of HCl from its feed solution, the HCl and its metal salts tend to move towards the water side due to the concentration difference across the membrane.
With the presence of an anion exchange membrane (AEM), the Cl- ions (or other anions like SO4 2-, NO3 -, PO4 3-, etc.) are allowed to pass through while the metals in the waste solution are less likely to pass.
Although the H+ ions are positively charged, they have higher competition in diffusion compared to metal ions because of their smaller size, lower valence state, and higher mobility. Therefore, they can diffuse along with the Cl- ions (or other anions) to maintain electrical neutrality.
The transport of H+ ions is a critical aspect of the DD process. Proper AEM properties are also necessary, including stability in acidic conditions, high H+ permeability, strong rejection for other metal ions, relatively high water uptake (WR), and poor water permeability.
Advantages of Utilizing Diffusion Dialysis