What Is Electrodeionization (EDI)?
Electrodeionization (EDI) is an advanced hybrid separation process combining ion-exchange resins with ion-exchange membranes to remove impurities from water. This process is widely used in industries such as electronics, pharmaceuticals, and power generation, where high-purity water is required for manufacturing processes.
The key advantage of EDI is that it produces high-purity water that is free from impurities, without the need for chemicals or regeneration of the resin. Unlike traditional ion exchange, EDI operates continuously, making it more efficient and cost-effective. Additionally, EDI systems are environmentally friendly, as they do not generate waste brine streams that need to be disposed of.
EDI systems can be designed to meet a variety of water treatment requirements, from small-scale laboratory applications to large-scale industrial applications. The level of water purity produced by the EDI process can be customized to meet the specific needs of the application.
The most widely used technique for generating ultra-pure water for applications in the microelectronics, pharmaceutical, and boiler feedwater industries is Electrodeionization (EDI). Typically, EDI is employed in conjunction with reverse osmosis (RO) to ensure that the feed water for EDI has a hardness level of less than 1 ppm.
How Does Electrodeionization (EDI) Work?
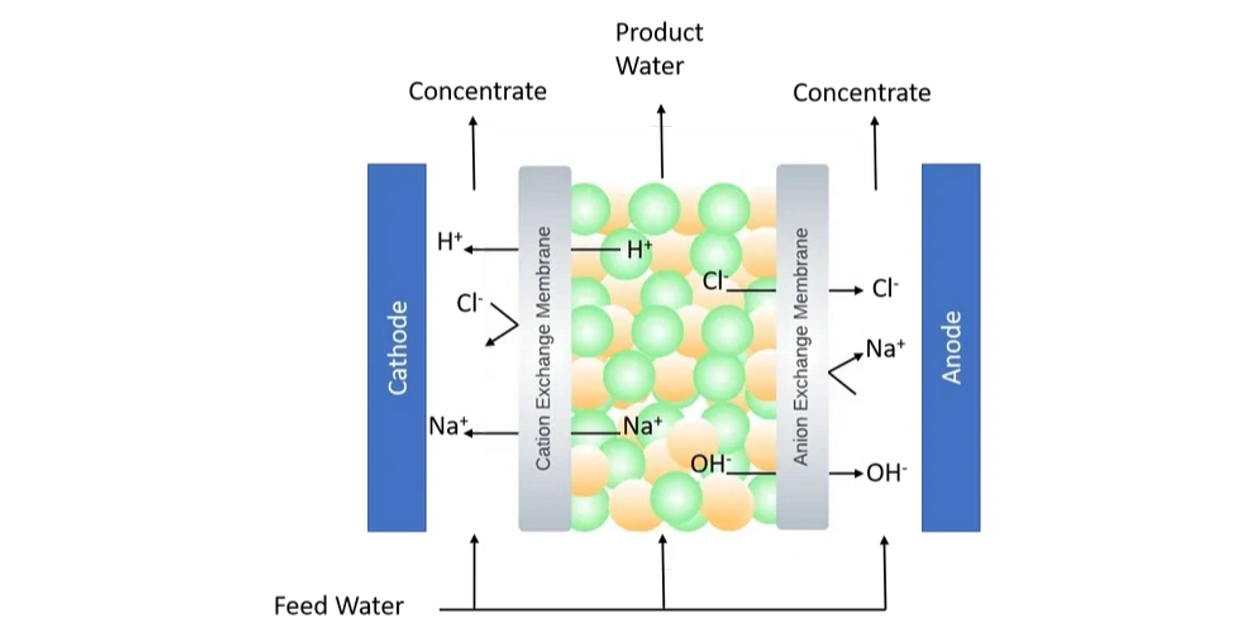
An electrodeionization (EDI) system comprises of a cation and an anion exchange membrane placed among the electrodes, much like in an electrodialysis (ED) system. The EDI system consists of concentrated and dilute chambers that are formed between the electrodes, where a succession of cation and anion-exchange membranes alternate.
To reduce resistivity and encourage anion and cation mobility under voltage difference, the dilution chamber of an EDI system is loaded with mixed resins. When feed water enters the dilution chamber and a potential difference is provided to the electrodes, cations migrate to the cathode, and anions go to the anode.
During the operation of the EDI system, water breakdown produces hydrogen and hydroxide ions. The generated protons and hydroxide ions can rejuvenate the resins, allowing for continued operation without the need for a chemical regeneration step. This feature makes EDI an environmentally friendly process as no chemicals are used to detach resins in the electrodeionization procedure.
The EDI technology does not require chemicals, and therefore, there is no need for scrubbers for stream neutralization after acid and base restoration, reservoirs for storing high concentration acids and alkaline solutions, associated hydraulic equipment and nozzles, and double pipes of rust metal, which greatly reduces both capital goods and operating expenses.
Most Common Applications Of EDI
Electrodeionization (EDI) is widely used in various industries for producing high-purity water, including:
- Desalination: EDI can be used in combination with other desalination techniques, such as Reverse Osmosis (RO), to produce high-quality drinking water from seawater or brackish water sources.
- Pharmaceutical Industry: In the pharmaceutical industry, EDI is used for the production of water that meets the strict quality standards of the US Pharmacopeia (USP) and the European Pharmacopeia (EP). The high-purity water is used as a solvent in the manufacturing of drugs and as a component in injection and infusion fluids.
- Power Generation: EDI is used in the power generation industry to produce ultrapure water that is free from impurities such as dissolved solids, silica, and carbon dioxide. The high-purity water is used as a coolant and as feed water for boilers and steam turbines.
- Semiconductor Manufacturing: In the semiconductor manufacturing industry, EDI is used to produce ultrapure water that is free from contaminants such as metals, particles, and organic impurities. The high-purity water is used in the fabrication of microchips and other semiconductor devices.
- Food and Beverage Industry: EDI is used in the food and beverage industry to produce water that is free from impurities such as chlorine, pesticides, and herbicides. The high-purity water is used in the production of soft drinks, beer, and other beverages.
- Cosmetics Industry: EDI is used in the cosmetics industry to produce high-purity water that is free from impurities such as bacteria, viruses, and endotoxins. The high-purity water is used as a solvent and a component in cosmetics and personal care products.
Overall, the most common use of EDI is for producing high-purity water in industries where water quality is critical for the manufacturing process or the end product.

